Orbital Notation For Sulfurs Electron Configuration
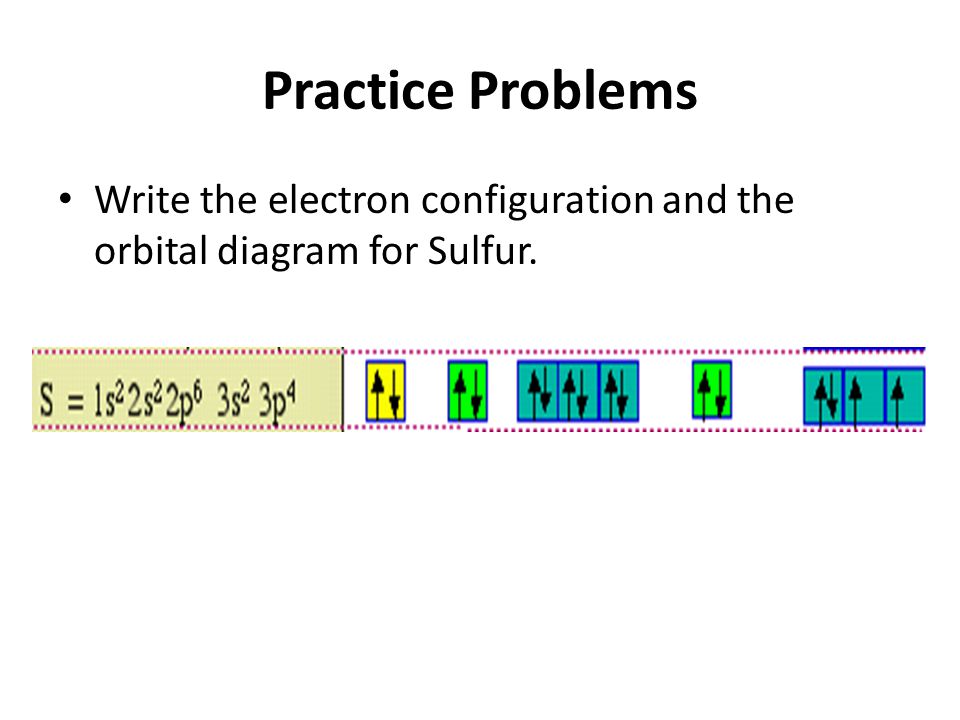
Write the Orbital Diagram Electron Configuration and Noble Gas Configuration for Phosphorus. Orbital Filling Diagram for Sulfur.
What Is The Electronic Configuration Of Sulfur Quora
Write the electron configuration.

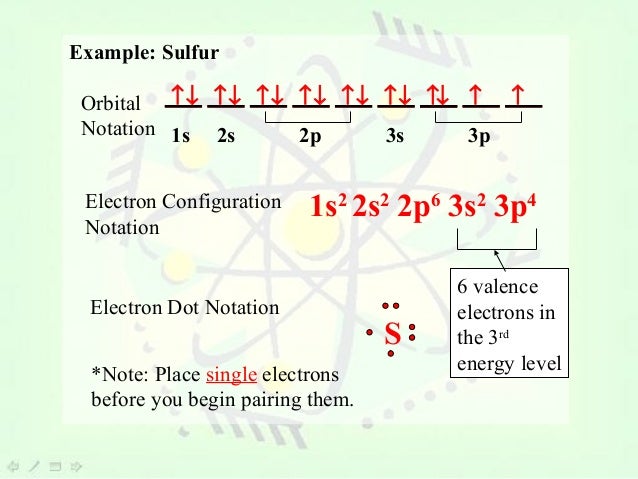
Orbital notation for sulfurs electron configuration. The electron configuration of sulfur is 1s2 2s2 2p6 3s2 3p4. Each arrow represents an electron. The arrows represent the 16 electrons of the sulfur atom and the directions represent their spins.
The orbital diagram for sulfur has seven boxes with two arrows pointing in opposite directions and two boxes with one arrow pointing up in each. Of electron in 2nf orbit 8 Max. 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 1.
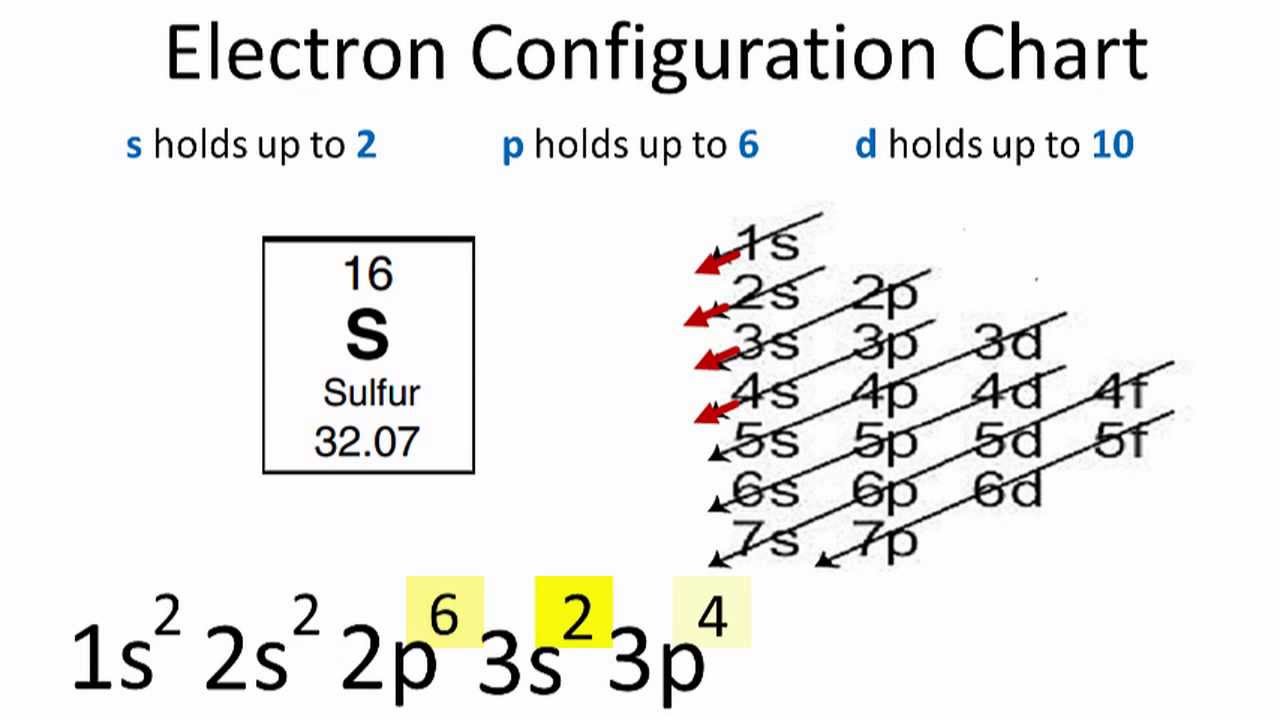
It is in the third period in the p block of elements. In SO2 sulfur is sp2. What Is The Orbital Configuration For Sulfur Quora Core electrons efficiently shield outermost electrons from nuclear chargeeffective nuclear charge increases as you move to the right across a row in the periodic table.
Capacityis 8 1st orbit has only 1s orbital. Orbital Notation is a way to show how many electrons are in anorbital for a given element. Number of Sulphur 16 No.
View desktop site What is the ground state electron configuration of sulfur whose atomic number is 16. The orbital notation for sulfur is. Sulfurs electron configuration is 1s 2 2s 2 2p 6 3s 2 3p 4.
One arrow represents one electron in a shell. Using the periodic table determine the electron configuration for sulfur. What is the orbital diagram for sulfur the orbital diagram for sulfur has seven boxes with two arrows pointing in opposite directions and two boxes with one arrow pointing up in each the arrows solved show the orbital filling diagram for s sulfur st answer to show the orbital filling diagram for s sulfur stack the sub shells in order of energy with the.
Twoarrows will be pointing differently. So for scandium the 1 st and 2 nd electron must be in 1s orbital the 3 rd and 4 th in the 2s the 5 th through 10 th in the 2p orbitals etc. Black Friday is Here.
The boxes represent sulfurs orbitals. In atomic physics and quantum chemistry the electron configuration is the distribution of electrons of an atom or molecule or other physical structure in atomic or molecular orbitals. Sulfur can bond to six atoms by promoting electrons into the 3d sublevel to give six unpaired electrons and the possibility of six bonds.
This is a memory device to remember the order of orbitals for the first two quantum numbers. Well we use the aufbau principle and for sulfur Z When we use an orbital notation for an elements electron configuration we are visually representing the probable locations of its electrons. The boxes represent sulfurs orbitals.
Write the electron configuration and draw the orbital notation for atoms of oxygen and sulfur. For example write the electron configuration of scandium Sc. Capacity is 8 No.
Capacity is 2 No. Count from left to right in the p block and you determine that sulfurs valence electrons have an ending configuration of 3p 4 which means everything up to that sublevel is also full so its electron. 1s²2s²2p⁴ The orbital diagram has five boxes with two arrows in the first three and single arrows in the last two.
Of electron in 2nf orbit 8 Max. Select the correct orbital diagram for this element. Electronic configurations describe each electron as moving independently in an orbital in an.
Since 1s can only hold two electrons the next 2 electrons for sulfur go in the 2s orbital. To see this video other videos chemistry education text and practice problems visit my website. Of electron in 3rd orbit 16-28 6 Max.
In order to write the Sulfur electron configuration we first need to know the When we write the configuration well put all 16 electrons in orbitals around the. In SF6 sulfur is sp3d2. They can either be shown with arrowsor circles.
One up and one down to show amaximum of two electrons with different spinElectron Configuration for Sulfur SWhat is the orbital notation of Sulfur. We start filling out the chart at the 1s orbital and work upwards. For example the electron configuration of the neon atom is 1s 2 2s 2 2p 6 using the notation explained below.
This is referred to as an expanded. Write the electron configuration and draw the orbital notation for atoms of oxygen and sulfur. 1s2 2s2 2p6 3s2 3p4 the number after the letter is the superscript orbital notation.
In SO42- sulfur is sp3. In writing the electron configuration for Sulfur the first two electrons will go in the 1s orbital. Start Your Numerade Subscription for 50 Off.
First locate sulfur in the periodic table. Write the electron configuration and draw the orbital notation for atoms of 0313 Write the electron configurations of the six cations that form from sulfur b. Of electron in 1st orbit 2 Max.
See full answer below. The next six electrons will go in the 2p orbital. The hybridization of sulfur will depend on what it is bonded to and how many atoms it is bonded to.
Sulfur Electron Configuration Notation The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. The p orbital can hold up to six electrons. An electron configuration lists only the first two quantum numbers n and ell and then shows how many electrons exist in each orbital.
What Is The Orbital Configuration For Sulfur Quora
 Electron Configuration For Chlorine Cl
Electron Configuration For Chlorine Cl
 Electron Configuration For Aluminium Al
Electron Configuration For Aluminium Al
 What Is The Orbital Notation For Sulfur Study Com
What Is The Orbital Notation For Sulfur Study Com
Electron Configurations How To Write Out The S P D F Electronic Arrangements Of Atoms Ions Periodic Table Oxidation States Using Orbital Notation Gce A Level Revision Notes
 Chapter 5 Electron Configurations
Chapter 5 Electron Configurations
 Electron Configuration And Orbital Diagrams Ppt Video Online Download
Electron Configuration And Orbital Diagrams Ppt Video Online Download
 Electron Configuration For Sulfur S
Electron Configuration For Sulfur S
 Find The Electron Configuration For Sulfur S And The Sulfide Ion S2 Youtube
Find The Electron Configuration For Sulfur S And The Sulfide Ion S2 Youtube
Posting Komentar untuk "Orbital Notation For Sulfurs Electron Configuration"